Введение
Хронический гастрит – это воспалительное заболевание слизистой оболочки желудка, характеризующееся повреждениями, степень и распространенность которых зависят от их этиологии и реакции организма. Инфекция, вызванная Helicobacter pylori (Hp), является наиболее распространенной причиной хронического активного гастрита во всем мире, химические вещества и аутоиммунные заболевания составляют небольшую долю хронических, чаще неактивных гастритов. Хронический гастрит эпидемиологически и биологически связан с развитием рака желудка, а Hp внесен в список канцерогенов I класса [1]. Эпидемиологические данные свидетельствуют о том, что степень, интенсивность и характер распространения воспаления и атрофии желудка тесно связаны с заболеваемостью раком желудка в популяции [2–4].
Основываясь на текущем понимании морфологических изменений при гастрите и их эволюции, полезно определять фенотипы хронического гастрита, основываясь на их классификации, при которой необходимо исследовать совокупную степень интенсивности воспалительных компонентов и стадию анатомической протяженности атрофически-метапластических изменений, связанных с риском развития рака [5]. Атрофия желудка, связанная с H. pylori, и кишечная метаплазия являются известными факторами риска развития рака желудка [6], было высказано предположение, что стадия гастрита может быть надежным показателем риска развития рака [7]. Поэтому необходимо оценивать результаты систем морфологического стадирования OLGA (Operative Link for Gastritis Assessment) и OLGIM (Operative Link for Gastric Intestinal Metaplasia) у всех пациентов с жалобами на желудочно-кишечную диспепсию.
Определение гастрита, вызванного H. pylori (Нр+), как инфекционного заболевания на Киотской конференции по глобальному консенсусу в январе 2014 г. вызывает обеспокоенность в борьбе с инфекцией H. pylori и ее серьезными последствиями по всему миру [8]. Лечение инфекции H. pylori приводит к быстрому исчезновению полиморфно-ядерной инфильтрации, за которой следует уменьшение хронического воспалительного инфильтрата с постепенной нормализацией слизистой оболочки. Эрадикация H. pylori осложняется постоянно растущей устойчивостью к антибиотикам, необходимостью тестирования на чувствительность к препаратам с учетом новых молекулярных технологий, тщательного выбора препаратов первой линии и вспомогательных средств. Также рассматривается роль H. pylori и антибиотиков и их влияние на микробиоту кишечника. Прогресс, достигнутый в лечении инфекции, вызванной H. pylori, был отражен в 6-м издании Маастрихтского/Флорентийского консенсусного доклада 2021 г. [9]. Атрофия слизистой оболочки и метапластические изменения практически не имеют обратного развития [10].
M. Haber и соавт. в своем обследовании пациентов с эрозивным эзофагитом, участвовавших в клинических испытаниях, показали, что от 75 до 90% H. pylori-отрицательных (Нр-) пациентов с диспепсией страдали гастритом [11]. Аналогичным образом у 56–69% Нр- пациентов с функциональной диспепсией или неэрозивным гастроэзофагеальным рефлюксом, участвовавших в клинических испытаниях, был диагностирован гастрит [12]. В этих исследованиях из антрального отдела и тела желудка брали 2 образца биопсии (т.е. по 4 образца биопсии на каждого пациента) и использовали окрашивание серебром по Уортину–Старри для выявления инфекции H. pylori. Биопсию тела желудка брали из середины большой кривизны тела желудка, а биопсию антрального отдела – из малой кривизны. Эти 2 исследования были относительно ограничены протоколами биопсии, основанными только на гистологическом исследовании, без учета предыдущего лечения H. pylori или потенциальных факторов, таких как курение, употребление алкоголя, нестероидных противовоспалительных средств (НПВС) или ингибиторов протонной помпы (ИПП) [13].
Данные, полученные в развитых странах, свидетельствуют о том, что распространенность гистологического гастрита снижается за последние десятилетия [3]. Это, вероятно, объясняется снижением распространенности инфекции H. pylori в разных странах мира [9]. Исследования, проведенные среди людей с симптомами и без них, которым была проведена плановая эзофагогастродуоденоскопия (ЭГДС), показали, что частота гастрита варьируется от 37 до 57% в популяции [14, 15]. Несмотря на то что Нр- гастрит стал чаще диагностироваться, его клиническое значение во многих случаях не до конца изучено.
Существует установленная связь между H. pylori и язвенной болезнью [16]. Однако связь с гастродуоденальными симптомами неясна, в амбулаторных исследованиях у H. pylori-положительных (Нр+) и H. pylori-отрицательных (Нр-) пациентов с желудочной диспепсией выявляются схожие жалобы и клинические проявления [17]. Симптомы со стороны верхних отделов желудочно-кишечного тракта (ЖКТ) являются частыми жалобами пациентов, однако лишь немногие из них имеют установленную органическую причину [18]. Врачи ошибочно связывают «эндоскопический гастрит» с симптомами в области живота, а гистологический гастрит определяют достаточно редко из-за того, что при выполнении ЭГДС эндоскописты часто не берут биоптаты для морфологического исследования [19].
В настоящее время имеются ограниченные данные о распространенности гистологических изменений в желудке у населения в целом [20–22]. Из имеющихся данных неясно, существует ли четкая связь между симптомами и эндоскопическими находками [23]. Однако поскольку большинство исследований, описанных в литературе, не были по-настоящему популяционными и не включали всестороннюю оценку гистологии, а Нр- гастрит в настоящее время встречается реже, связь между различными формами гистологического гастрита и симптомами часто не уточнялась.
В настоящее время доступны серологические маркеры, связанные с заболеваниями желудка, в т.ч. пепсиноген-I (PGI), пепсиноген-II (PGII), PGI/PGII (PGR), гастрин-17 (G17) и антитела к H. pylori (H. pylori IgG). Эти серологические маркеры могут помочь выявить людей с предраковыми заболеваниями, которым необходимо пройти гастроскопию [25]. Примечательно, что эти биомаркеры желудка уже более 20 лет используются для проведения серологической биопсии слизистой оболочки желудка с целью выявления предраковых состояний и рака желудка. Однако в современных исследованиях основное внимание уделяется не только PGI, соотношению PGI/PGII и H. pylori IgG, но и другим биомаркерам, таким как G17 и PGII.
Пепсиноген – это неактивный предшественник пепсина, специфического функционального фермента в слизистой оболочке желудка, который изначально был обнаружен при язве двенадцатиперстной кишки [25]. Он вырабатывается клетками слизистой оболочки желудка и в зависимости от иммуногенности существует в двух вариантах: PGI и PGII. PGI в основном вырабатывается главными клетками фундальных желез желудка, которые представляют собой слизистые клетки. PGII может происходить из слизистых клеток в антральном отделе желудка, а также верхней части двенадцатиперстной кишки. Бόльшая часть пепсиногена секретируется в желудок, а небольшая его часть (примерно 1%) поступает в кровоток, где его содержание всегда стабильно [26]. Число пепсинродуцирующих клеток в слизистой оболочке желудка отражает соотношение PGI/PGII в сыворотке крови, а также косвенно указывает на морфологическое состояние различных участков слизистой оболочки желудка и ее пепсинсекреторную функцию [27]. При увеличении секреции желудочной кислоты увеличивается уровень PGI, и наоборот [25]. Секреторные участки выработки PGII и факторы, влияющие на его уровень, относительно многочисленны, а его повышение связано с атрофией фундальных желез, эпителиальной метаплазией, дисплазией и некоторыми другими изменениями [28]. Поэтому для определения состояния слизистой оболочки желудка можно проводить совместный мониторинг PGI и PGII. Соответствующие данные мониторинга PGI/PGII также важны для серологической биопсии слизистой оболочки фундальных желез желудка. Некоторые ученые считают, что повышение уровня PGII в сыворотке крови напрямую связано с изменениями слизистой оболочки желудка, вызванными инфекцией H. pylori, что важно для отличия нормальной слизистой оболочки желудка от аномальной [28]. В некоторых исследованиях сравнивалось содержание пепсиногена у пациентов, инфицированных H. pylori, до и после лечения. Содержание PGI после успешной эрадикации значительно ниже, чем до лечения, а содержание PGII снижается еще сильнее, соответственно, соотношение PGI/PGII значительно увеличивается. Определение содержания пепсиногена имеет некоторую клиническую ценность при оценке эффективности эрадикации H. pylori. Но новые исследования показывают, что длительный прием ИПП может вызвать побочные эффекты в организме вплоть до увеличения риска смерти [29–31]. Поэтому в клинической практике лечения H. pylori следует внимательно следить за применением ИПП, особенно при длительном лечении. Не найдено приемлемых результатов в отношении других оцениваемых факторов (PGI и G17).
G17 тесно связан с воспалением, иммунитетом, инвазией и метастазированием раковых клеток желудка и слабо экспрессируется у здоровых людей. При патологических изменениях, таких как гастрит, рак желудка и т.д., уровень G17 значительно повышается [32]. Однако по мере прогрессирования заболевания желудка диагностическая ценность сывороточного G17 для атрофического гастрита на поздних стадиях снижается [33]. Примечательно, что увеличение или уменьшение экспрессии G17 может указывать на риск развития рака желудка [34]. Сам по себе G17 не является прямым фактором, вызывающим рак желудка, но он может способствовать развитию некоторых канцерогенных факторов, активировать множественные сигнальные пути, оказывать антиапоптотическое и противовоспалительное действие, а также стимулировать секрецию желудочной кислоты, что приводит к онкогенезу [35].
Цель работы: оценить клинико-лабораторные и гистологические изменения слизистой оболочки желудка при Нр+ и Нр- гастритах.
Материалы и методы
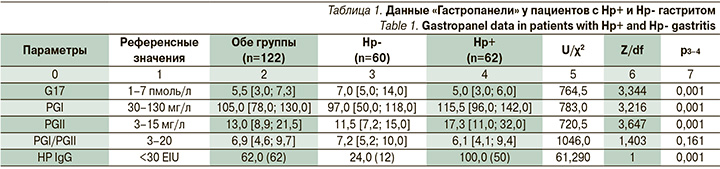
Были обследованы 122 пациента, из которых 62 имели гастрит Нр+ и 60 человек Нр- гастрит. Возраст пациентов колебался от 20 до 65 лет и был сопоставим в обеих группах, соотношение женщин и мужчин в обеих группах было также сопоставимо – 4:1 соответственно. Пациенты с язвенной болезнью желудка и двенадцатиперстной кишки и другими язвенными поражениями гастро-дуоденальной зоны, а также с аутоиммунным гастритом не включались в исследование. Каждого пациента подробно опрашивали и осматривали на предмет выявления коморбидной патологии. В лабораторные исследования, кроме общепринятых клинических и биохимических анализов, была включена «Гастропанель» – комплекс лабораторных исследований крови посредством технологии иммуноферментного анализа (ИФА), который позволяет неинвазивно оценить функциональное и анатомическое состояние слизистой оболочки желудка. В «Гастропанели» исследуются: базальный G17, PGI, PGII, оценка соотношения уровней PGI и II, антитела IgG к H. pylori [36, 37]. Для диагностики хеликобактериоза использовали классический уреазный дыхательный тест, основанный на определении 13С-мочевины и исследование кала на наличие антигенов H. pylori с применением моноклональных антител.
Всем пациентам назначали эндоскопическое исследование верхних отделов ЖКТ с взятием биоптатов и морфологическим исследованием их по критериям OLGA/OLGIM [5, 38, 39] и с проведением быстрого уреазного теста (Хелпил-тест) для диагностики Нр. Фактически у каждого пациента поиск Нр-инфекции проводился с помощью двух или трех методик.
У участников исследования оценивали вегетативный статус, контрольную группу составили 32 здоровых человека сопоставимых пола и возраста. Определение исходного вегетативного тонуса в покое проводили посредством клинической оценки и анализа ритмограммы в покое и вегетативной реактивности в активном ортостазе – по методу математического анализа ритма сердца Р.М. Баевского (1979) и таблицам Вейна–Соловьевой (1981). Для анализа ритмограммы и клинической оценки вегетативного статуса использована оригинальная компьютерная программа «КОРВЕГ» (авторы Е.Ю. Плотникова, Э.И. Белобородова и соавт.) [40].
Всем пациентам назначали лечение гастрита в зависимости от наличия Нр-инфекции в соответствие с актуальными отечественными клиническими рекомендациями [41]. Пациентам с Нр+ гастритом проводили тройную терапию в комбинации с висмутом трикалия дицитратом в течение 14 дней, после курса антихеликобактерной терапии (АХТ) применяли пробиотики и ребамипид до двух месяцев. Пациентам с Нр- гастритом применяли ИПП, ребамипид, по показаниям – пробиотики.
Исследование проведено в соответствии с принципами Хельсинкской декларации Всемирной медицинской ассоциации (в редакции 2000 г. с разъяснениями, данными на генеральной ассамблее ВМА, Токио, 2004), с правилами Качественной клинической практики Международной конференции по гармонизации (ICH GCP), этическими принципами, изложенными в Директиве Европейского союза 2001/20/ЕС и требованиями национального российского законодательства. От каждого пациента было получено информированное согласие на участие в исследовании.
Обработку полученных данных проводили с использованием пакета прикладных программ Statistica ver.10 (StatSoft, Inc), графическое отображение результатов анализа выполнено в программе MS Excel 2010 (Microsoft Corporation). Вид распределения количественных показателей проводили при помощи критерия Колмогорова–Смирнова с коррекцией Лильефорса, по результатам оценки все данные имели распределение, отличное от нормального. Количественные показатели представлены медианой, 25 и 75 процентилем (Me [LQ; HQ], качественные – в виде частот (процентов). Оценку различий между группами проводили с использование непараметрических критериев, сравнение 2 групп проводили с помощью критерия Манна–Уитни, 3 и более групп – критерия Краскела–Уоллиса. Если результаты теста Краскела–Уоллиса были статистически значимы, проводили тест Данна. Критический уровень значимости нулевой статистической гипотезы принимали равным 0,05.
Результаты
Согласно литературным данным, с возрастом распространенность гастрита увеличивается, но различий между полами не наблюдается, при этом в течение жизни у 50% испытуемых слизистая оболочка желудка претерпевает изменения – формируется хронический гастрит [42]. В нашем исследовании симптомы со стороны верхних отделов ЖКТ наблюдались почти у всех обследуемых пациентов обеих групп, изжога несколько чаще наблюдалась в группе с Нр+ гастритом, но статистически значимых различий мы не получили.
Уменьшение распространенности гастрита в развитых странах [43] согласуется с уменьшением распространенности инфекции H. pylori [44]. В свою очередь увеличивается число случаев Нр-гастрита, хотя его значение до конца не определено. Данные свидетельствуют о том, что Нр- гастрит чаще поражает одну часть желудка по сравнению с гастритом, вызванным H. pylori [45]. Эти данные соответствовали нашим результатам: Нр- гастрит чаще верифицировался в антральном отделе, в то время как Нр+ гастрит почти всегда поражал как антральный отдел, так и тело. А метаплазия по OLGIM 0-I наблюдалась только у 9 пациентов с Нр+ гастритом.
По данным «Гастропанели», в нашем исследовании у 3/4 всех пациентов с Нр- гастритом был отрицательный результат на антитела к Нр (табл. 1). В нашем исследовании 12 пациентов, серопозитивных к Hp (24%), страдали Нр- гастритом, что указывало на экс-Нр+ гастрит, хотя факт АХТ они отрицали.
Причинами H. pylori-негативного гастрита в нашем исследовании могли быть следующие.
- Предыдущая инфекция H. pylori, которая не беспокоила пациентов и не диагностировалась, вероятно, была вылечена на фоне курсов антибиотикотерапии по различным причинам. Однако серологические тесты на Нр IgG, использованные в нашем исследовании, остаются положительными в течение длительного времени после эрадикации H. pylori. В одном исследовании через 3,5 года после успешного лечения H. pylori у 72% пациентов сохранялись положительные результаты Нр IgG [46]. Следовательно, транзиторная инфекция Нр или ее самоэрадикация была обнаружена нами с помощью ИФА.
- Прием ИПП. В исследовании 68% пациентов с H. pylori-негативным гастритом либо ранее, либо в настоящее время принимали ИПП, а у 54% были гистологические признаки приема ИПП [47]. В исследованиях на животных Y. Zavros и соавт. показали, что длительный прием ИПП может привести к воспалению желудка, возможно, из-за чрезмерного роста микроорганизмов [48]. Кроме того, в предыдущих исследованиях H. pylori-негативного гастрита длительное лечение лансопразолом было связано со снижением остроты и хронизации воспаления тела желудка и антрального отдела [28]. Также возможно, что ИПП являются основным средством контроля симптомов изжоги при гастроэзофагеальной рефлюксной болезни, которая была выявлена у половины Hp- пациентов.
- Инфекция, вызванная другими микроорганизмами, кроме H. pylori (например, Mycobacterium avium-intracellulare, Herpes simplex или Cytomegalovirus в, которые могут проникать в слизистую оболочку желудка и вызывать воспаление). Однако эти инфекции крайне редко встречаются у пациентов без нарушенного иммунитета, но все участники нашего исследования перенесли коронавирусную инфекцию 2019-nCoV, которая могла негативно отразиться на их иммунитете. В задачи нашего исследования не входил поиск других инфекционных причин гастрита.
- Химический или реактивный гастрит, часто вызванный рефлюксом желчи или приемом НВПС. Почти все пациенты с H. pylori-негативным гастритом изредка (но не курсом) однократно по требованию принимали НПВС. Однако прием НВПС обычно вызывает повреждения с минимальным воспалением, и их можно легко отличить при гистопатологическом исследовании.
Результаты «Гастропанели» у всех пациентов не выходили за рамки референсных значений. Не было выявлено существенных различий в уровне секреции G17 у людей разных пола, возраста и индекса массы тела (p>0,05). Уровни G17 и PGII у H. pylori-положительных пациентов были значимо ниже, чем у H. pylori-отрицательных пациентов (p>0,001). Уровни PGI и PGII и их соотношение прямо пропорционально соотносились с другими показателями «Гастропанели» (табл. 1). Так как у всех пациентов не было выраженных гистологических изменений, то секреторные функции желудка у них были сохранены. При этом, по оценке вегетативного статуса, подавляющее число пациентов обеих исследуемых групп имели ваготонию в положении покоя, в отличие от контроля (p>0,05). Вегетативная реактивность статистически значимо (p>0,05) отличалась от контроля, особенно по асимпатической реактивности. Эти данные указывают на преобладание влияния вагуса у пациентов с гастритом, независимо от наличия или отсутствия Нр.
Всем пациентам с Нр+ гастритом назначали АХТ, выбор ИПП в схеме лечения оставался за пациентом. Ряд пациентов выбирал комбинированный препарат, содержащий 20 мг омепразола (капсула на прием), 500 мг кларитромицина (таблетка на прием) и 500 мг амоксициллина тригидрат (2 капсулы на прием) с коммерческим названием Пилобакт® АМ (Ранбакси Лабораториз Лимитед, Индия). Каждый стрип, содержащий таблетки и капсулы набора Пилобакт® АМ рассчитан на один день лечения и состоит из двух частей: красной – с надписью «утро» и синей – с надписью «вечер». Пациент, пользующийся данным набором, проглатывал таблетки и капсулы целиком. Продолжительность лечения составляла 14 дней. Как правило, к утреннему и вечернему приему через полчаса добавляли висмута трикалия дицитрат в дозе 240 мг. Эффективность Пилобакта® АМ для АХТ оценивалась в нескольких исследованиях, результаты которых отражены в табл. 2.
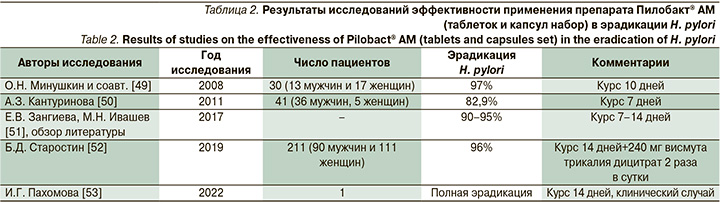
Приведенные нами исследования демонстрируют, что уровень эрадикации на фоне применения препарата Пилобакт® АМ не отличался от уровня эффективности АХТ по российским данным из Европейского регистра Helicobacter pylori (Hp-EuReg) – 81,1% при 10-дневном и 86,7% при 14-дневном курсе [54]. Эффективность АХТ при применении Пилобакта® АМ в вышеприведенных исследованиях была более 90% при курсе 10–14 дней. Пациенты в нашем исследовании, которым проводили АХТ с применением Пилобакта® АМ и висмута трикалия дицитрата в течение 14 дней, имели уровень эрадикации 87,4%. Такой уровень, вероятно, был связан с определенной резистентностью Нр к антибиотикам.
Мы исследовали фармакохимические свойства препаратов, входящих в состав Пилобакта® АМ. Для исследования применяли следующие методы:
- Инфракрасная спектроскопия (ИК-спектроскопия) – это научный метод, используемый для анализа и идентификации химических веществ на основе того, как они взаимодействуют с инфракрасным светом. Он включает в себя измерение поглощения, испускания или отражения инфракрасного излучения молекулами, предоставляя ценную информацию об их структурах и составе.
- Высокоэффективная жидкостная хроматография (ВЭЖХ) – это метод анализа, используемый в химии, фармацевтике, биохимии, анализе окружающей среды и других областях. Он позволяет разделять и идентифицировать компоненты сложных смесей, а также количественно определять их содержание.
Амоксициллин и кларитромицин, входящие в состав Пилобакта® АМ, мы сравнивали с референсными препаратами с помощью метода ИК-спектроскопия. Результаты представлены на рис. 1.
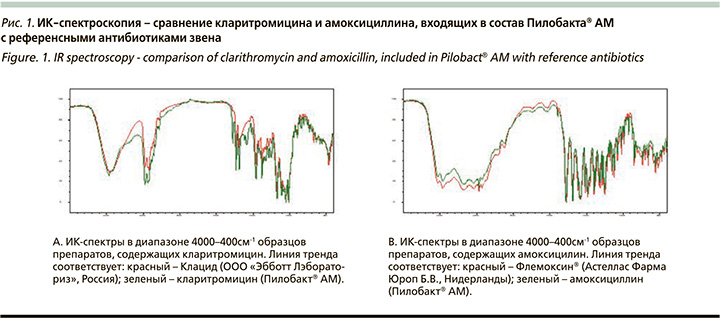
Основные полосы поглощения двух образцов кларитромицина сопоставимы по основным полосам поглощения. Наличие полос поглощения при 1775, 1616, 1586 см-1 соответствует индивидуальным особенностям спектра Пилобакта® АМ. Сопоставимость ИК-спектров по кларитромицину – 83%. Полосы поглощения амоксициллина достаточно соотносятся между рассматриваемыми образцами, сопоставимость спектров составляет более 98%, эти препараты оказались практически идентичны. Таким образом, антибиотики, особенно амоксициллин, входящие в состав Пилобакта® АМ, продемонстрировали очень высокую фармакохимическую сопоставимость с референсными препаратами, что указывает на их потенциальную сопоставимость и в клиническом эффекте. Небольшие различия ИК-спектров, видимо, связаны с некоторой разницей вспомогательных веществ, входящих в состав препаратов.
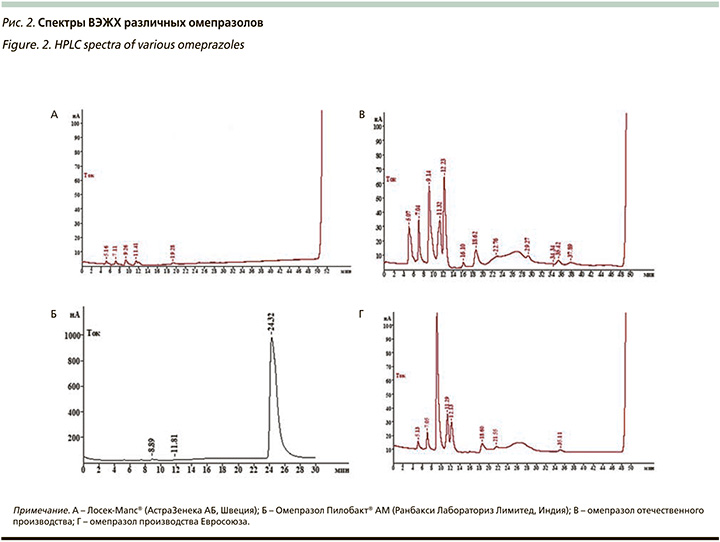
Омепразол, входящий в состав Пилобакта® АМ, мы подвергли ВЭЖХ, при которой у нас была возможность сравнить непосредственно «чистоту» самого омепразола без вспомогательных веществ. В нашем исследовании мы сравнивали спектры ВЭЖХ оригинального омепразола (Лосек-Мапс®, АстраЗенека АБ, Швеция), омепразол (Пилобакт® АМ) и еще 2 омепразола – один российский, а другой европейский генерики. Результаты исследования представлены на рис. 2. Элюция омепразола из Пилобакта® АМ (Б – рис. 2) происходила на 24,32 минуты и по активности в условиях амперометрического детектирования соответствует силе сигнала около 989 нА. Сила отклика примесных компонентов на 8,89 и 11,81 минуты соответствовала 14,3 и 10,05 нА. Данное обстоятельство с учетом высокой чувствительности амперометрического детектора позволяет говорить о следовых количествах примесных компонентов. Не было выявлено практически никаких отличий между образцами А и Б (рис. 2) – омепразолы оригинальный и из Пилобакта® АМ. В образцах сравнения В и Г (рис. 2) в условиях ВЭЖХ примесные компоненты обладали значительной активностью – компонент элюирующийся около 8,96 минуты обладал откликом около 1193 нА, а компонент с элюцией 11,67 минуты – 387 нА, элюция основного вещества в данных условиях хроматографии наблюдалась на 25,8 минуты. Таким образом, ВЖЭХ омепразолов продемонстрировала, что «чистота» омепразола, входящего в состав Пилобакта® АМ не отличалась от оригинального препарата, но была значительно выше, чем в других исследуемых гененриках.
Заключение
Хотя считается, что основной причиной хронического гастрита является инфекция, вызванная H. pylori, наше исследование продемонстрировало, что Нр- гастрит при морфологическом исследовании встречается чаще, чем считалось ранее. При этом значимые различия в клинико-лабораторных показателях у пациентов с Нр+ Нр- гастритах почти не выявляются. Эти результаты подчеркивают необходимость более тщательного изучения гистологии слизистой оболочки желудка при этих желудочно-кишечных заболеваниях. Распространенность гастрита, вызванного H. pylori, в нашем исследовании соответствовала тенденции к ее снижению в развитых странах. А одним из антихеликобактерных вариантов лечения может использоваться препарат Пилобакт® АМ, который доказал свою высокую эффективность как в клинических наблюдениях, так и фармакохимическую сопоставимость с референсными препаратами.



